FREE Shipping on all our products! (Please Note: Orders may experience a delay of a week or more in shipping due to the high volume of orders at this time of year.)
FREE Shipping on all our products! (Please Note: Orders may experience a delay of a week or more in shipping due to the high volume of orders at this time of year.)
3 Hands-on Science Activities to Explore the Chemistry of Dry Ice
April 01, 2019 3 min read
Hi-ya! We're back!!
After a bit of a hiatus and a move, the Sassafras Twins are back in hou-ouse! We are feeling out our new digs and plan on sharing a few things with you all over the coming months.
We have plenty of out of this world fun planned ahead. But for today, we are bringing back everybody's favorite uncle, our eccentric, but loveable Uncle Cecil, to share a bit of chemistry fun with you all!
And here he is...
The Science of Dry Ice

Howdy-hoody Sassy-Sci peeps! And a big wave to all you other sciency-friends!!
(President Lincoln is insisting that I put a link to my previous posts in case you don't know who we are. So, here you go.)
The Prez and I love to get a chunk of dry ice to play with! It's one of those materials that appear to boil in room temperature water, sending out billows of white, wispy smoke, which is super helpful when we are practicing for a Floating Tomatoes concert.
Today, we wanted to share three hands-on science activities from our lab to yours that you can use to explore this amazing material. But before we do that, let's look at the science behind dry ice...
Dry ice is frozen carbon dioxide, the same chemical that can be found floating around in our atmosphere. Dry ice is often used to keep things cold for a long period of time because its temperature is around -109.3°F (-78.5°C).
What makes dry ice unique is that fact that it readily sublimes at standard atmospheric pressure and temperature.
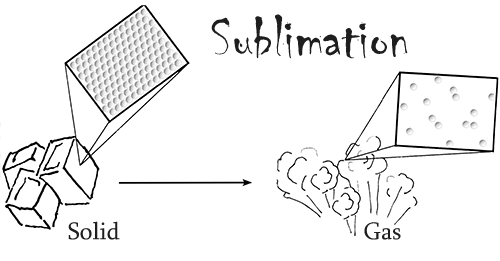
In other words, under normal conditions, this material changes from a solid directly into a gas, skipping the liquid phase. Dry ice also has three times the cooling power per volume of regular ice.
Due to these two facts, this chemical is often used in transporting materials that need to stay refrigerated over a long period of time.
Okie-dokie, enough with the science - let's get to the fun!!
3 Science Activities to Explore Dry Ice
To do these activities, you will need the following:
- Dry Ice
- 2 Plastic Cups
- Water
- Dish soap
- 2 Plates
- Regular Ice
Dry Ice in Water
Start by filling a clear plastic cup halfway with water. Then, place a chunk of dry ice in the cup and observe what happens.
You should see bubbles immediately form in the liquid. As the bubbles rise to the top, they release a milky white gas, which is composed of water vapor and carbon dioxide.
Dry Ice with Soap
Next, fill a clear plastic cup halfway with water. Add 2 TBSP of dish soap to the water and mix well. Then, add a chunk of dry ice in the cup and observe what happens.
You should see bubbles immediately form in the liquid. Although this time, there will be far more than in the first activity. In fact, they will most likely grow out and over the container because the soap has trapped the gases, preventing them from immediately escaping. As the bubbles pop, they release the same milky white gas as seen before.
Disappearing Ice
Begin by selecting two small, but equal pieces of dry ice and of regular ice. Place each piece on a separate plate and set the plates aside. After 45 to 60 minutes check the two plates and observe what has happened.
You should see that the two ice cubes have disappeared, but the regular ice cube left behind a puddle of water, whereas the plate with the dry ice cube is empty. This is because frozen water melts into a liquid at room temperature, while frozen carbon dioxide sublimes into a gas. This is why we call frozen carbon dioxide, “dry ice.”
A Few More Dry Ice Activities
Well, hopefully, you get a chunk of solid carbon dioxide to play with in the near future. President Lincoln and I also wanted to share the following website for more ideas!
We'll be back in a few months with more science fun, until then, you can read more about Train's and Blaisey's journey with me in the Sassafras Science Adventures Series!
Also in Homeschool Science Activities
Don’t Be Afraid To Try These 3 Christmas Tree Experiments Right Now
December 07, 2024 3 min read

It's the most wonderful time of the year and these three Christmas tree experiments will make your season even brighter! Click "Read More" to see them.
Steps For Dissecting A Seed By Felipe Moreno
November 09, 2024 2 min read

Felipe Moreno, one of the Sassafras twins' botany experts, shares an Argentinian folk ballad which shares the steps for dissecting a seed.
How to make a Beautiful Fall Leaf Book {A Fall Science Activity}
September 21, 2024 2 min read

Want to preserve the beauty of fall for science? Click "Read More" to learn how to make a fall leaf book and download the free templates!
Subscribe
Sign up to get the latest on sales, new releases and more …

