FREE Shipping on all our products! (Please Note: Orders may experience a delay of a week or more in shipping due to the high volume of orders at this time of year.)
FREE Shipping on all our products! (Please Note: Orders may experience a delay of a week or more in shipping due to the high volume of orders at this time of year.)
The Periodic Table - A Brief Explanation of one of the Foundations of Chemistry
June 15, 2020 5 min read

The periodic table visually shows the elements that make up everything we see around us. It is a key concept in chemistry that needs to be taught to our students. In fact, we recommend that you introduce the periodic table in the elementary years.
{Listen to this episode below}
But it can be a bit confusing because it appears as if there are so many different visual options out there...
One periodic table will be full of color, dividing elements into families and giving them names, like the one below.
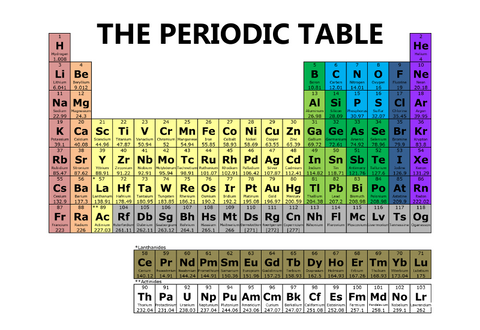
One periodic table will have only three colors, like the one below.
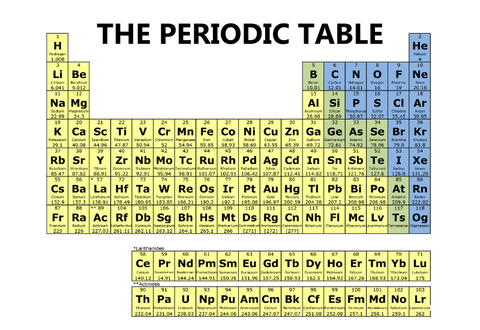
One periodic table will have four different blocks, like the one below.
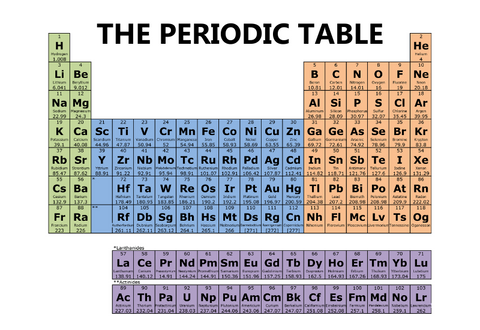
And yet another periodic table will have columns, or groups, of different colors, like the one below.
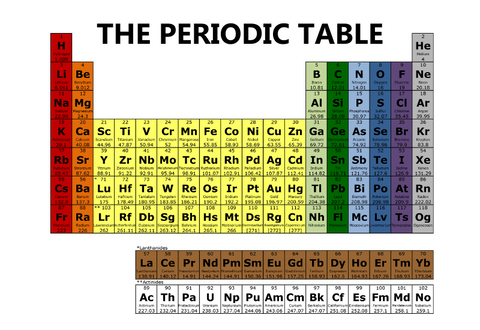
And there are even more options for visual representations of the periodic table than the four we have shared here.
Add to that the differences in where and how the inner transition metals - the lanthanides and actinides - are shown. Plus, elements have been added and named over the years, poor hydrogen doesn't seem to know what group it belongs to, and selenium is sometimes a nonmetal, sometimes a metalloid!
All this begs the simple question - which periodic table is the right one?
Well . . . in some way . . . they all are.
I know - that's clear as muddy water!!
But remember that the periodic table is a way for us to visually show the relationships between the elements. Just like our human relationships, these elemental relationships can be defined, or shown, in different ways.
Plus as technology grows, our ability to more clearly define those relationships grows as well. And as we experiment with the elements, we discover new things that help to shape the periodic table.
So, while a newer periodic table may depict new elements we have created and a deeper understanding of the relationships between elements - it doesn't make an older version wrong. It's just a different snapshot of our understanding of the elements.
When you consider this, it's okay to look at different versions and appreciate what they can share with us. Each "picture" helps us to learn more about how elements are related to each other.
So, with this "snapshot" idea as the backdrop, how do we as educators go about sharing the periodic table with our students?
Let's begin with a short explanation of what the periodic table does tell us...
The Periodic Table - A Brief Explanation
As I said before, the periodic table shows the relationship between the elements.
It is a systematic arrangement of the known and created elements in order of increasing atomic number. And, it is designed so that elements with similar properties together are grouped together.
In other words, you can tell at a glance which elements will behave similarly and which ones will be wildly different in how they react.
The periodic table typically gives the following information for each element…
- The atomic number, which is the number of protons that can be found in the nucleus of an atom.
- The atomic mass, which is the total weight of the protons, neutrons, and electrons in a given atom. Sometimes this can vary if there are isotopes of the element, so the atomic mass given on the periodic table is an average of those varying weights. (Note - Typically, those bracketed atomic masses mean that the number is an estimate. The elements with these brackets are very unstable or recently discovered.)
- The chemical symbol, which is the 1-, or 2-letter code that scientists use for the element. This code is accepted internationally to remove language barriers when discussing chemical compounds. Some are easy, like O for Oxygen; some make less sense, like Pb for Lead. This is because the symbol is typically based on the Latin name for the element, which in the case of lead is plumbum. Chemists use the symbol of an element when referring to it in a compound or equation, so these are important to know.
As you move from left to right on the table, the atomic number and atomic mass of the element increases. The same is true as you travel down the periodic table.
The history of the periodic table
The original periodic table was created by Russian chemist Dmitri Mendeleev. He wrote it almost 30 years before Thomson discovered the electron, close to 45 years before Rutherford found the nucleus of an atom and over 50 years before scientists determined that the proton and neutron made up the nucleus of the atom!
Mendeleev proposed a primitive version of today’s periodic table as he was writing a textbook on general chemistry. Through his research he was struck by the fact that the elements chemical properties varied with the atomic mass, so he drew up a table to show these relationships.

Mendeleev’s 1871 Periodic Table, photo credit
In a stroke of genius, he left gaps for elements that had not yet been discovered and even went so far as to predict the properties of those missing elements. And amazingly, when these elements were finally discovered the properties were very similar to what Mendeleev had predicted!
Even though our modern-day table looks quite a bit different from what Mendeleev drew, we still give him credit for the original idea of the periodic table.
How we can share the periodic table in our homeschool
Here's how we share the periodic table throughout the years:
- In the elementary years, I introduce the idea of the periodic table, along with a simplified view of the groups.
- During the middle school years, I teach the basic relationships that the periodic table can show us, along with the periods and groups.
- And finally, for the high school years, the student can focus on learning the chemical principles and mathematics that the periodic table shows us.
Teaching this foundation of chemistry in this manner allows our students to learn about the elements and the periodic table at a level they will understand as they build upon it throughout the years.
You can introduce the periodic table informally through books and then use games to help your students learn the included elements, or you can choose to study chemistry more formally using a pre-planned program. Below are several of the options we have used along the way for homeschool science.
The following books are a few of my favorites to learn more about the periodic table:
- Basher Science: The Periodic Table (Kingfisher)*
- The Periodic Table (Scholastic)*
- The Mystery of the Periodic Table (Living History Library)*
-
The Elements: A Visual Exploration of Every Known Atom in the Universe (Theodore Gray)*
*(The above links are affiliate links.)
Here are two games to help your students learn more about the elements of the periodic table:
- Periodic Table Match-up {Free Download}
- Periodic Table Battleship {Directions to set-up and play the game are in the linked post. Here is a free periodic table for you to use with the game.}
And to help your kiddos memorize the periodic table here is a song:
- The Periodic Table Song {updated in 2018}
Finally, if you choose to use a program to learn more about the periodic table, we offer the following programs to help you out:
- Science Chunks: Periodic Table - A simple unit study on the periodic table for ages K to 6th grade.
- The Sassafras Science Adventures Volume 7: Chemistry - A journey through the periodic table with the Sassafras Twins.
- Chemistry for the Grammar Stage - A first introduction into the world of chemistry for your elementary students.
- Chemistry for the Logic Stage - A second look at the world of chemistry best for middle school students.
- High School Chemistry - Free plans for high school chemistry.
No matter how you choose to share the periodic table with your students, it will serve to increase their understanding of chemistry deepen their appreciation of the elements that make up the world all around us!
Also in Elemental Science Blog
How can we use one experiment for multiple ages?
June 06, 2025 4 min read

Is it possible to do only one experiment for homeschool science each week with multiple kids? Yes, but it will take some work. Click to get tips on how.
All About Experiment Variables for Hands-on Science and Science Fair Projects
April 12, 2025 2 min read

What variables are found in an experiment? And how can you know which is which? Click "Read More" to get the answers.
4 Easy Steps to Add Current Events into your Homeschool Science Plan
November 02, 2024 3 min read

Adding a current events study to a subject can increase the student’s interest as well as inform him or her of the latest advances in the field. Having a student read a relevant article or two each month will enhance what he or she is learning in a subject.
This month, I wanted to share with you all how to incorporate the latest scientific news into your studies.
Subscribe
Sign up to get the latest on sales, new releases and more …

